Observing a Substance Before and After a Change
Posted by Joshua Bartosiewicz- Assessing and reading the lab write up.
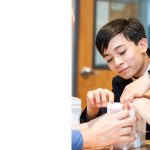
- Beginning to measure their baking soda.
- Precisely measuring the correct amount of baking soda for the experiment.
- Adding the baking soda to the plastic baggie.
- Using the triple beam balance to accurately measure their dry substances.
- Using a beaker to measure their liquid substances.
- Measuring their dry ingredients on the triple beam balance.
- Combining ingredients into the ziploc bag.
- The bag is inflating due to gas being released in the chemical reaction of the two substances mixed together
The 8th grade is in the latter half of our chemistry unit. After our young scholars have been taught the basics of chemistry such as the states of matter (liquids, solids, gasses, plasma), elements, atoms, and molecular structures, we have started diving into chemical reactions, chemical equations and natural vs synthetic materials. In these two labs, students had various substances such as road salt, sugar, baking soda, water, and vinegar which were combined into different mixtures. Students would observe the contents of each mixture before the reaction took place, which they noted in their lab write-ups. Once the initial analysis was recorded, students then added a final substance to their mixtures which would either act as catalyst (a catalyst is a substance that initiates a chemical reaction) and start a reaction, or cause nothing to happen at all. Students found that their Ziploc bags with road salt, baking soda and water created an exothermic reaction (a reaction that produces heat) where carbon dioxide was a product. We also concluded that mixing baking soda and vinegar together created a chemical reaction where carbon dioxide was also a product. Since the students had these mixtures in Ziploc bags which were closed after everything was mixed, they knew a gas was being released due to the bag blowing up like a balloon and the bubbles that were produced! In the end of both labs, students saw multiple indicators (heat production, gas production, bubbling) that tell us a chemical reaction has occurred.
← Building Bridges Through Language! Bantustan Breakdown →